Trisodium citrate Stock Solution
This guide describes the preparation of Trisodium citrate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Trisodium Citrate (C₆H₅O₇Na₃) Solution
Trisodium citrate (also called sodium citrate) is a white crystalline salt that dissolves readily in water. It serves as a buffering agent, chelating agent, and pH regulator in biochemical applications. A 1 M stock solution is conveniently prepared by dissolving the appropriate mass of trisodium citrate dihydrate in water and adjusting to the desired final volume.
Objective
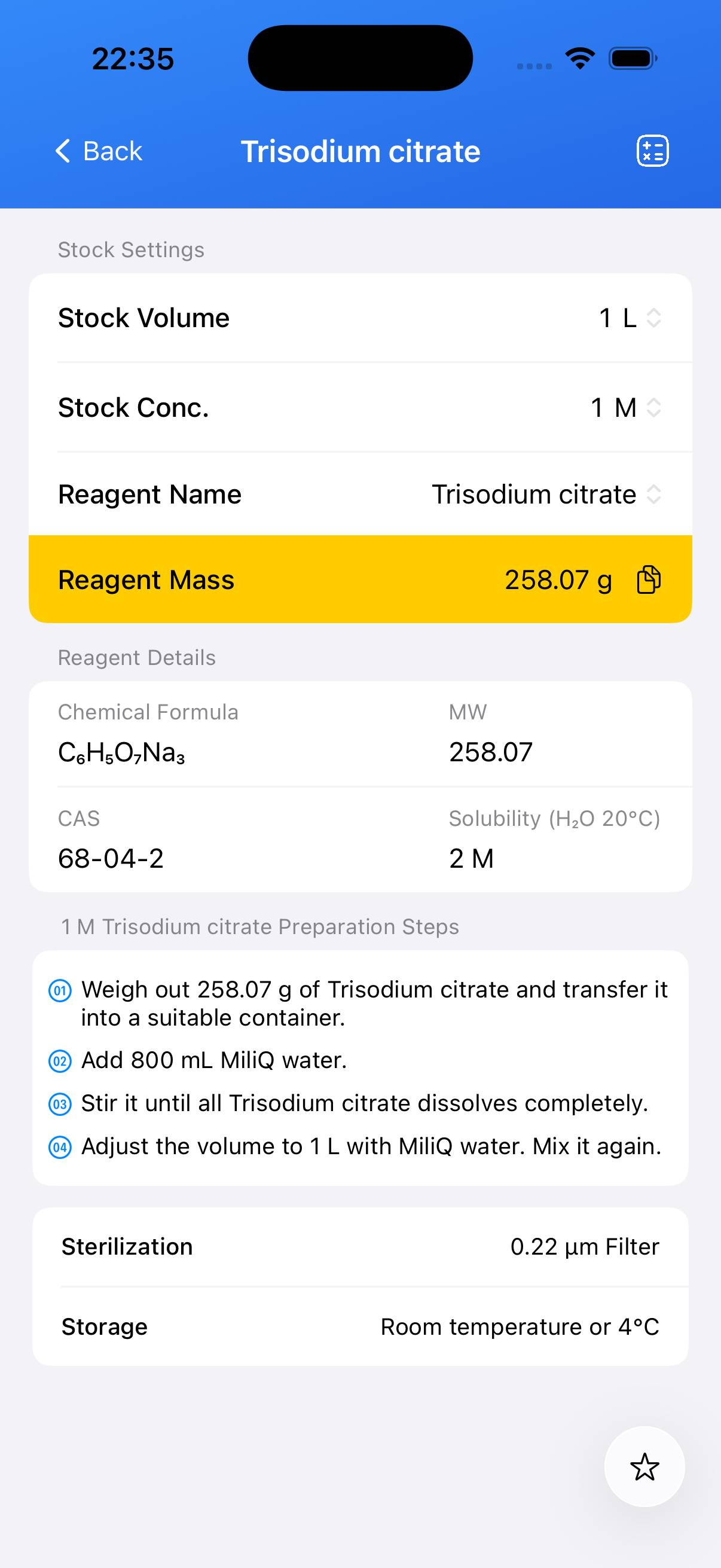
Preparation of a 1 M trisodium citrate stock solution.
Composition
- Trisodium citrate (C₆H₅O₇Na₃) aqueous solution of defined molarity
Required Materials
- Trisodium citrate or trisodium citrate dihydrate solid
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Stirring rod or magnetic stirrer
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
Determine the amount of solid required by molarity and hydration state. For a **1 M solution** using **trisodium citrate dihydrate (MW ≈ 294.1 g/mol)**: weigh **294.1 g** and scale proportionally for other volumes (e.g., 29.41 g for 100 mL). - Step 2
Add the weighed solid into a beaker containing approximately 80 % of the final desired volume of deionized water. Stir until completely dissolved. - Step 3
Transfer the dissolved solution into a volumetric flask. Rinse the beaker with deionized water to ensure all solute is transferred. Add water to reach the final volume. Mix thoroughly. - Step 4 (Optional)
If sterile solution is required, filter through a **0.22 µm membrane** filter into a sterile container.
Note: Trisodium citrate solutions are typically near neutral to slightly alkaline. Label the stock solution container with concentration and preparation date. Store in a sealed container at room temperature or per your lab’s protocol.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Trisodium citrate | C₆H₅O₇Na₃ | 258.07 | 68‑04‑2 |
| Trisodium citrate dihydrate | C₆H₅O₇Na₃·2H₂O | 294.10 | 6132‑04‑3 |