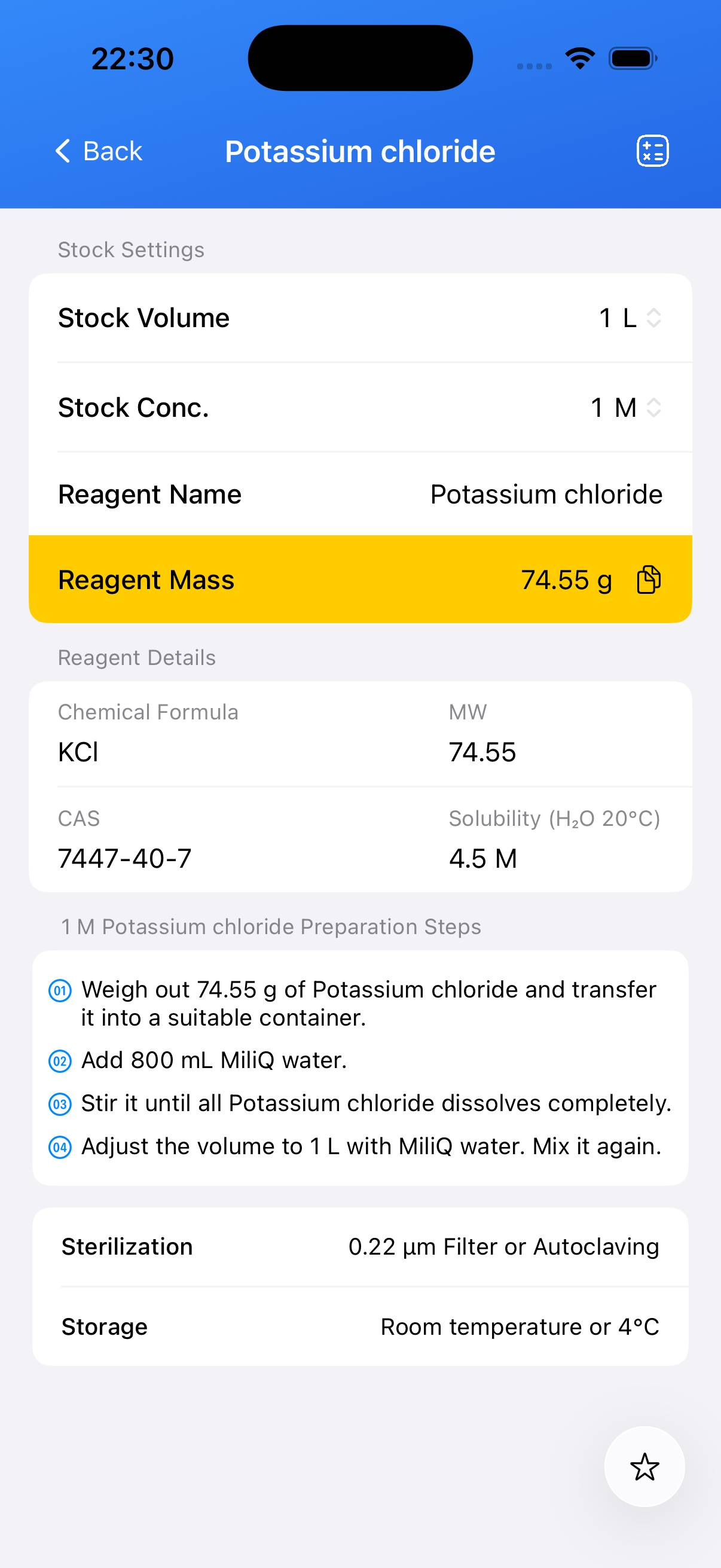
Potassium chloride Stock Solution
This guide describes the preparation of Potassium chloride Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Potassium Chloride (KCl)
Potassium chloride (KCl) is a simple inorganic salt widely used in biological, biochemical, and chemical laboratories. It dissolves readily in water and is commonly used to adjust ionic strength, prepare saline solutions, and formulate buffer systems. The molar mass of potassium chloride is 74.55 g/mol.
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Potassium chloride | KCl | 74.55 g/mol | 7447-40-7 |
Preparation of a 1 M KCl Solution
The following is a typical laboratory procedure to prepare 1 L of 1 M potassium chloride stock solution:
- Calculate the mass needed: 1 mol × 74.55 g/mol = 74.55 g of KCl.
- Weigh out 74.55 g of KCl using an analytical balance.
- Place the KCl into a beaker or volumetric flask and add approximately 700–800 mL of deionized or distilled water.
- Stir until the KCl is completely dissolved.
- Transfer the solution to a 1 L volumetric flask and add deionized water to bring the volume to exactly 1000 mL.
- Mix the solution thoroughly. The 1 M KCl stock is ready for use.
Notes and Precautions
- Ensure accurate weighing of KCl to achieve correct molarity.
- Add the solid to a portion of water first, then dilute to the final volume for best accuracy.
- Wear appropriate personal protective equipment (lab coat, gloves, goggles).
Storage and Usage
- The prepared 1 M KCl solution can be stored at room temperature or refrigerated if needed.
- Common applications include buffer preparation (e.g., PBS), saline solutions, and use as an ionic strength adjuster in biochemical assays.