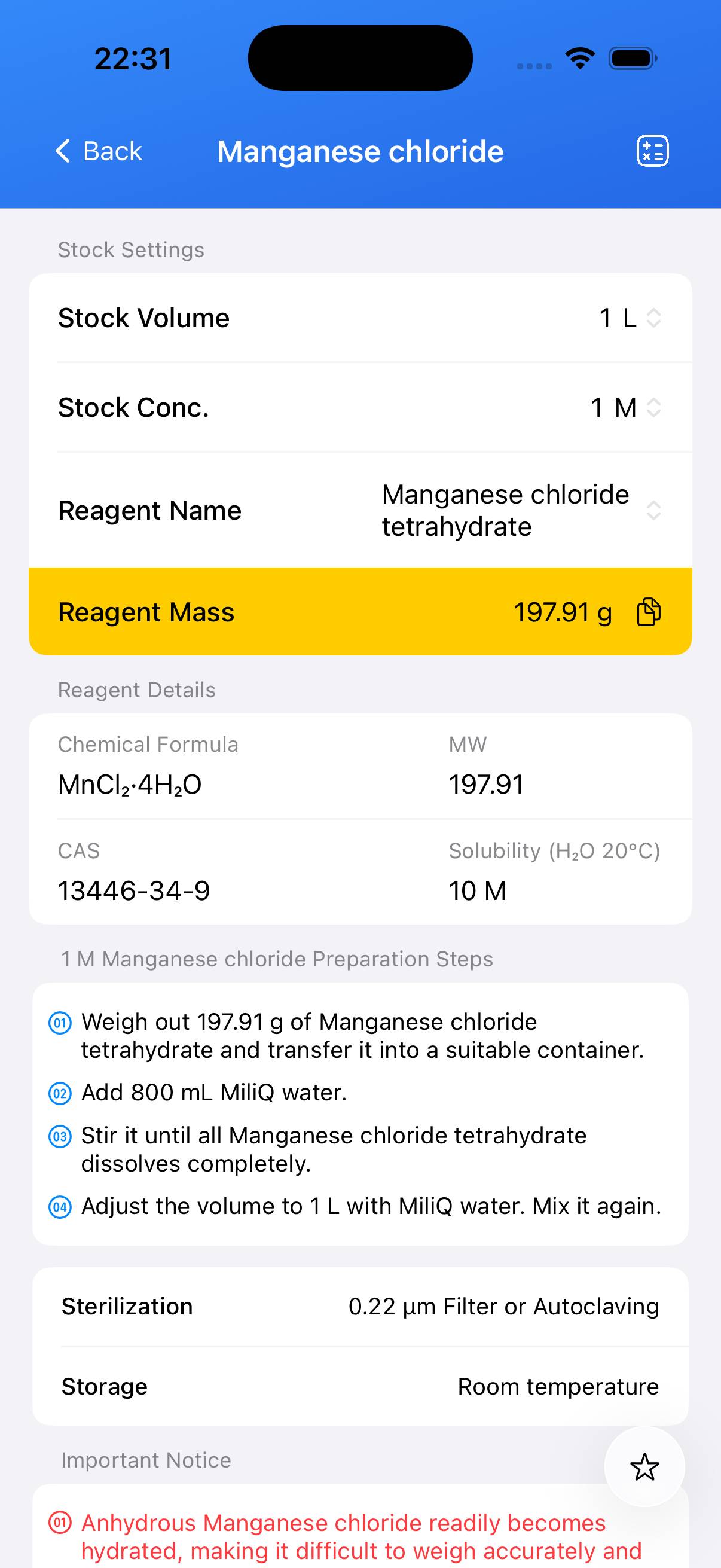
Manganese chloride Stock Solution
This guide describes the preparation of Manganese chloride Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Manganese(II) Chloride (MnCl₂)
Manganese(II) chloride (MnCl₂) is an inorganic salt that exists in anhydrous, dihydrate, and tetrahydrate forms. The hydrated forms are pink and readily dissolve in water to give solutions of Mn2+ ions.
Commercial solutions such as 0.1 M MnCl₂ in water are available for laboratory use.
Objective
Preparation of 100 mL of 0.1 M Manganese(II) chloride stock solution.
Composition
- 0.1 M Manganese(II) chloride (MnCl₂) aqueous solution
Required Materials
- Manganese(II) chloride reagent (anhydrous or hydrated)
- Deionized / distilled water
- 100 mL volumetric flask or graduated cylinder
- Analytical balance
- Mixing tool (magnetic stirrer or stirring rod)
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
Follow your laboratory’s safety protocols. Manganese compounds can irritate skin and mucous membranes; handle with appropriate PPE.
- Step 1
Calculate the required mass of MnCl₂ solid: Mass = concentration × volume × molecular weight. For 0.1 M and 0.1 L using **anhydrous MnCl₂** (MW ≈ 125.84 g/mol):
Mass ≈ 0.1 mol/L × 0.1 L × 125.84 g/mol = **1.26 g**. - Step 2
Accurately weigh ~1.26 g anhydrous MnCl₂. If using dihydrate (MnCl₂·2H₂O) or tetrahydrate (MnCl₂·4H₂O), adjust according to their respective molecular weights. - Step 3
Add the solid into ~80 mL deionized water and stir until fully dissolved. - Step 4
Transfer the solution to a 100 mL volumetric flask and bring to volume with deionized water. Mix thoroughly.
Storage: Label the stock solution with concentration and date. Store at room temperature or refrigerated according to your lab practices.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Manganese chloride tetrahydrate | MnCl₂·4H₂O | 197.91 | 13446-34-9 |
| Manganese chloride dihydrate | MnCl₂·2H₂O | 161.874 | 38639-72-4 |
| Manganese chloride (anhydrous) | MnCl₂ | 125.844 | 7773-01-5 |