Magnesium acetate Stock Solution
This guide describes the preparation of Magnesium acetate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Magnesium Acetate ((C₂H₃O₂)₂Mg) Solution
Magnesium acetate is a magnesium salt of acetic acid that dissolves readily in water and can be used as a source of magnesium ions or as a buffering agent. In laboratory settings, concentrated stock solutions (e.g., 1 M) are prepared by dissolving the appropriate mass of magnesium acetate in water and adjusting to the final volume. The tetrahydrate form is often chosen for accurate weighing.
Objective
Preparation of a defined concentration stock solution of magnesium acetate (e.g., 1 M).
Composition
- Magnesium acetate ((C₂H₃O₂)₂Mg) solution of defined molarity
Required Materials
- Magnesium acetate tetrahydrate or anhydrous solid
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Stirring rod or magnetic stirrer
- Filter unit (0.22 µm) for sterile applications (optional)
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
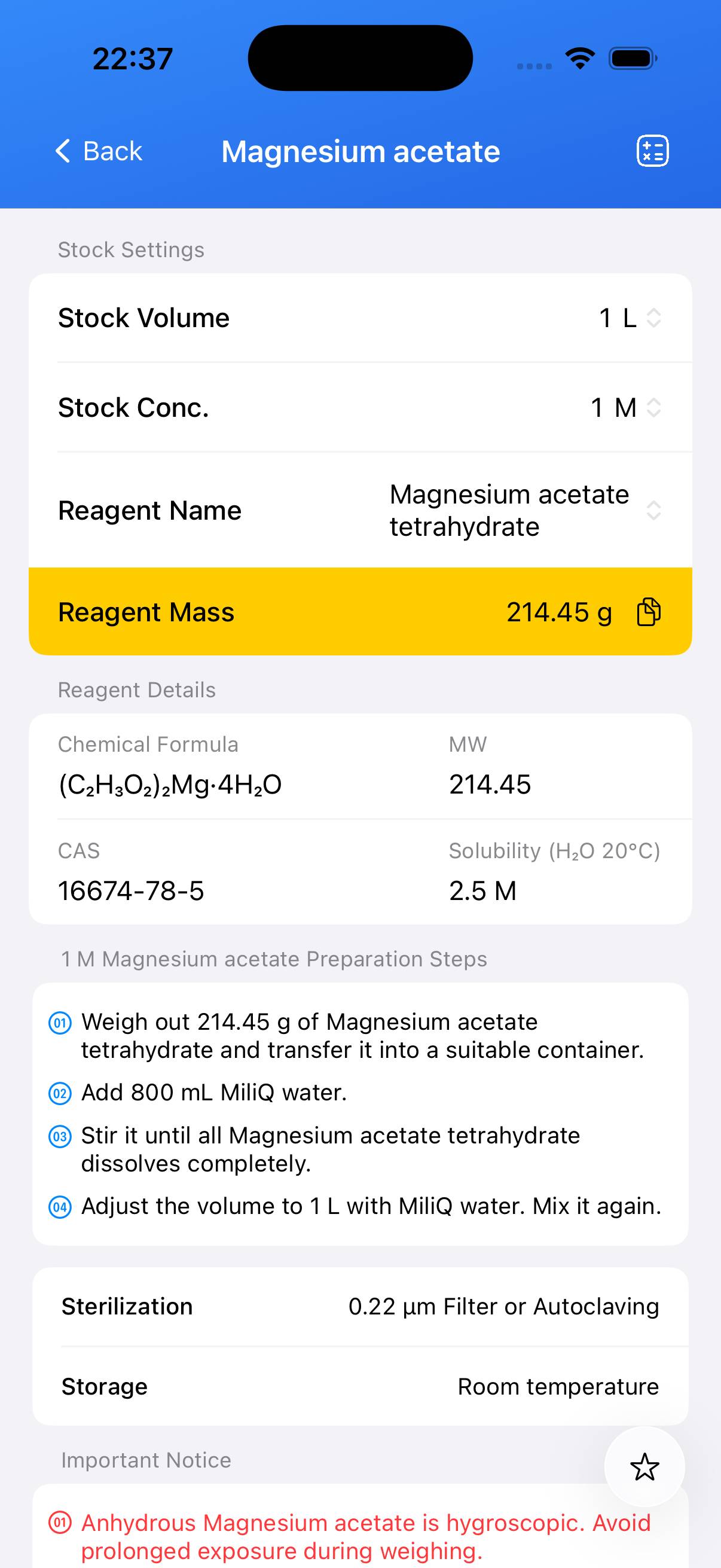
Determine the required mass for the target molarity. For a 1 M stock solution using **magnesium acetate tetrahydrate (MW ≈ 214.45)**: weigh **214.45 g** for 1 L. Scale proportionally for other volumes (e.g., 21.445 g for 100 mL). - Step 2
Place the weighed solid into a beaker containing approximately **80 %** of the final desired volume of deionized water. Stir until the solid dissolves completely. - Step 3
Transfer the solution to a volumetric flask. Rinse the beaker with water and add the rinses to the flask. Add deionized water to reach the final volume and mix thoroughly. - Step 4 (Optional)
If sterile stock solution is required, filter through a **0.22 µm** membrane into a sterile container. Heat sterilization is usually not necessary for magnesium acetate stock solutions.
Note: Magnesium acetate solutions are typically clear and colorless. Label the container with concentration and preparation date. Store the stock solution at room temperature or as specified by your protocol.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Magnesium acetate tetrahydrate | (C₂H₃O₂)₂Mg·4H₂O | 214.45 | 16674‑78‑5 |
| Magnesium acetate (anhydrous) | (C₂H₃O₂)₂Mg | 144.41 | 142‑72‑3 |