Preparation Glycine-Cl Stock Solution
This guide describes the preparation of Glycine-Cl Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Glycine‑Cl (Glycine Hydrochloride) Solution
Glycine‑Cl (glycine hydrochloride) is the hydrochloride salt of glycine, a simple amino acid. It is widely used in the preparation of Glycine‑HCl buffer systems, particularly at low pH ranges (commonly pH ≈ 2.2–3.6), suitable for affinity chromatography elution, immunoassays, and other applications requiring acidic buffer conditions.
Objective
Preparation of a defined concentration stock solution of Glycine‑Cl (e.g., for buffer preparation).
Composition
- Glycine‑Cl (glycine hydrochloride) aqueous solution at specified molarity
Required Materials
- Glycine‑Cl (glycine hydrochloride) solid
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Stirring rod or magnetic stirrer
- pH meter or pH strips
- Personal protective equipment (gloves, goggles, lab coat)
Procedure – Stock Solution
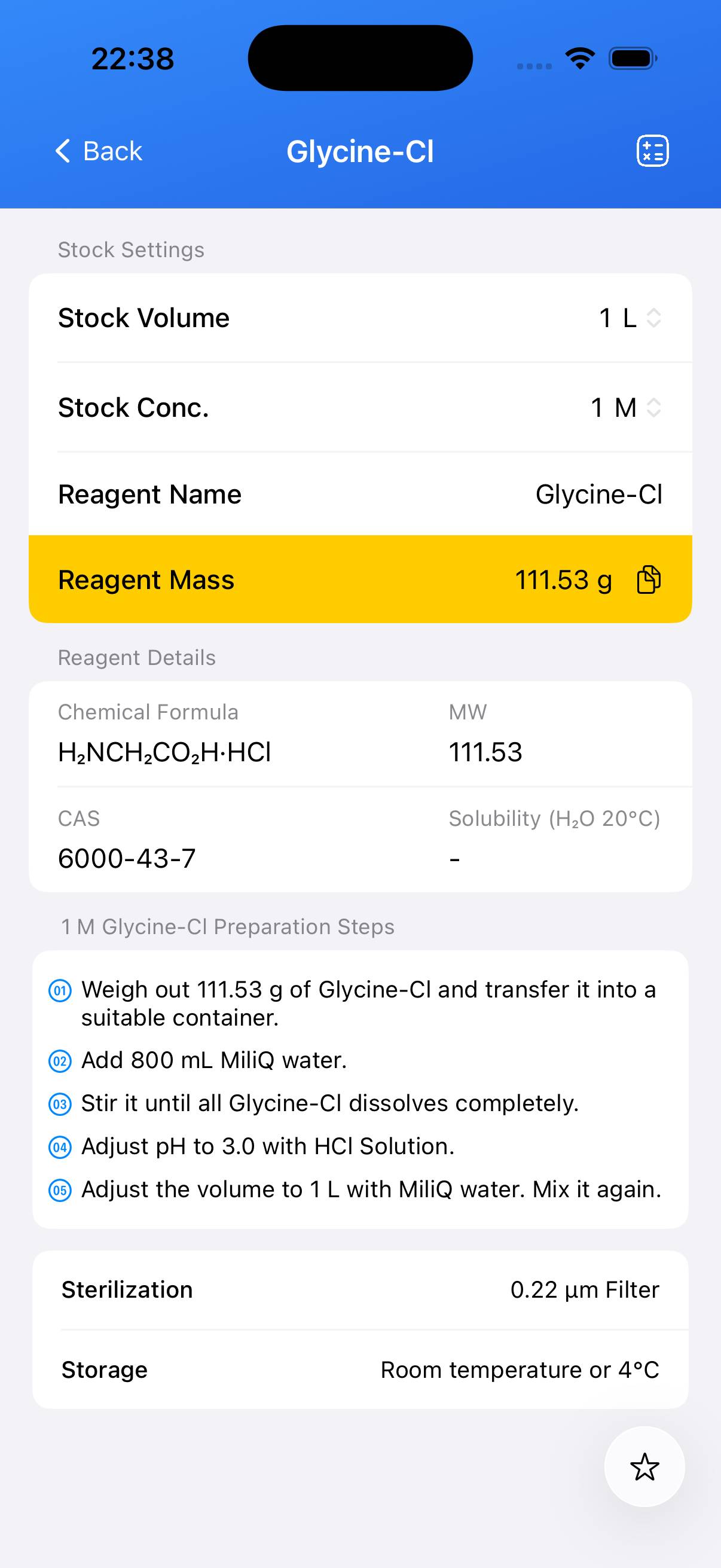
- Step 1
Decide your target stock molarity. For example, to make a **1 M Glycine‑Cl stock**: calculate the required mass using the molecular weight (111.53 g/mol). Weigh the calculated amount (e.g., **111.53 g** for 1 L of 1 M stock). - Step 2
Dissolve the weighed Glycine‑Cl solid in a beaker containing ~80 % of the target final volume of deionized water and stir until completely dissolved. - Step 3
Transfer the dissolved solution to a volumetric flask and rinse the beaker with water into the flask. Add deionized water to bring the solution to the final desired volume and mix thoroughly.
Procedure – Glycine‑HCl Buffer Preparation (Example)
To prepare a buffer at a specific pH (e.g., **0.1 M Glycine‑HCl, pH ~3.0**), you can use the Glycine‑Cl stock in combination with HCl or adjust pH directly in solution.
- Prepare an appropriate concentration of Glycine‑Cl stock (e.g., 0.1 M) by dissolving Glycine‑Cl in water.
- Adjust pH to the target value (e.g., pH 3.0) by adding HCl or NaOH while monitoring with a calibrated pH meter until the desired pH is reached.
- Once pH is set, transfer to a volumetric flask and adjust to final volume with deionized water.
Note: Glycine‑HCl buffers are commonly used in low pH applications such as protein elution and immunoassays. Label the stock solution with concentration and date, and store at room temperature or refrigerated as needed.
Available Reagents
| Name | Formula | MW | CAS |
|---|---|---|---|
| Glycine‑Cl (Glycine hydrochloride) | H₂NCH₂CO₂H·HCl | 111.53 | 6000‑43‑7 |