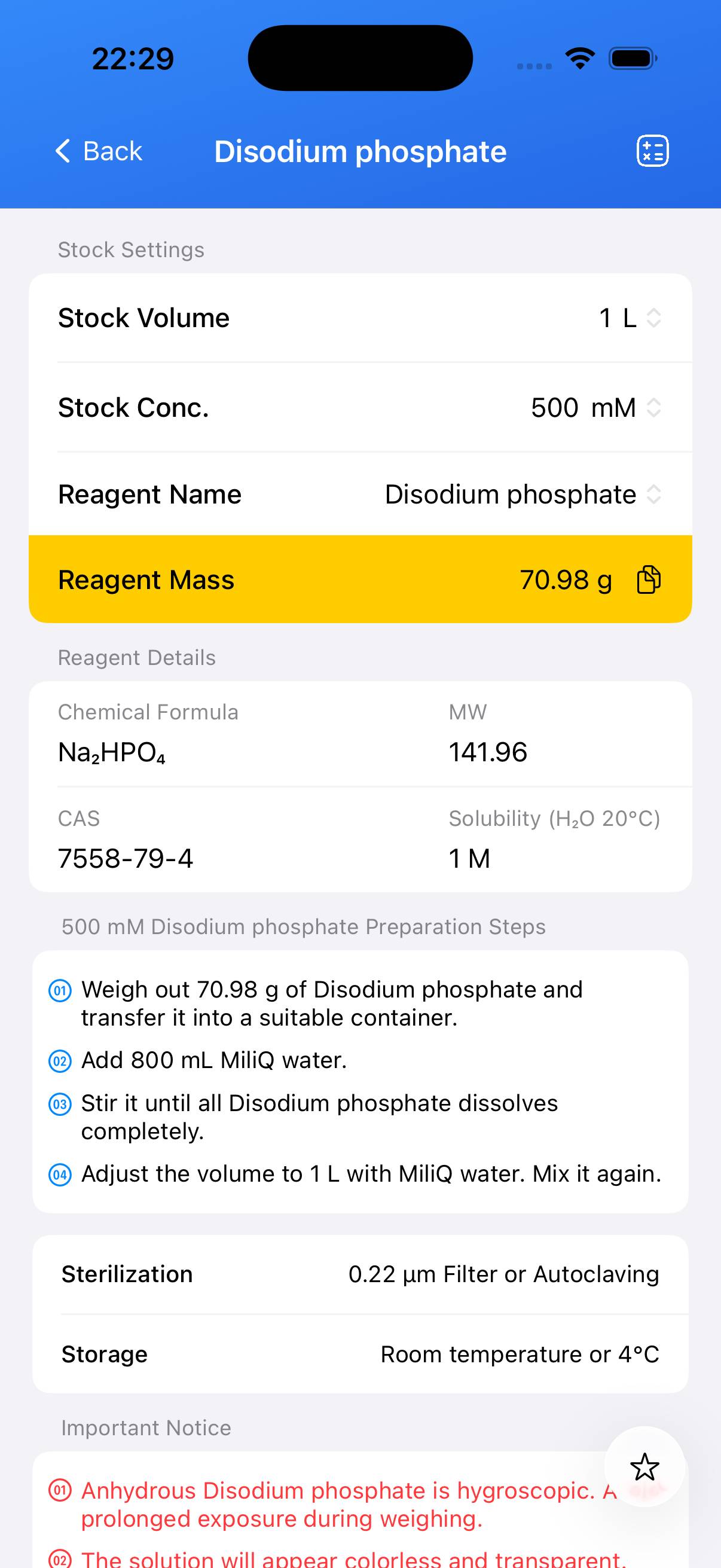
Disodium phosphate Stock Solution
This guide describes the preparation of Disodium phosphate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Sodium Phosphate Dibasic
A 1 M sodium phosphate dibasic stock solution can be prepared from sodium phosphate dibasic dihydrate (Na₂HPO₄·2H₂O). This solution is commonly used as a component in phosphate buffer systems or other biological applications requiring phosphate ions.
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Disodium phosphate (anhydrous) | Na₂HPO₄ | 141.96 g/mol | 7558-79-4 |
| Disodium phosphate dihydrate | Na₂HPO₄·2H₂O | 177.99 g/mol | 10028-24-7 |
| Disodium phosphate heptahydrate | Na₂HPO₄·7H₂O | 268.07 g/mol | 7782-85-6 |
| Disodium phosphate dodecahydrate | Na₂HPO₄·12H₂O | 358.14 g/mol | 10039-32-4 |
Preparation of 1 M Sodium Phosphate Dibasic
To prepare 1000 mL of 1 M sodium phosphate dibasic solution:
- Weigh out 177.99 g of sodium phosphate dibasic dihydrate (Na₂HPO₄·2H₂O).
- Transfer the solid to a 2 L beaker or flask.
- Add ~800 mL of deionized or distilled water and stir until completely dissolved.
- Adjust the volume to 1000 mL with deionized water and mix thoroughly.
- Solution will appear clear and colorless.
- If needed for sterile applications, transfer to an autoclavable bottle and autoclave (20 min at 121–124 °C, liquid cycle).
Notes and Precautions
- The hydrated dibasic phosphate is hygroscopic and may absorb water; adjust the weighed mass accordingly if necessary.
- Do not dissolve solid directly into 1000 mL water; large solute addition can increase final volume.
- Wear appropriate personal protective equipment (lab coat, gloves, goggles).
Storage and Usage
- The prepared 1 M solution can be stored at room temperature when not in use.
- This stock is commonly used to prepare phosphate buffer solutions when mixed with monosodium phosphate stock at desired ratios.
Preview