Dipotassium phosphate Stock Solution
This guide describes the preparation of Dipotassium phosphate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Dipotassium Phosphate (K₂HPO₄)
Dipotassium phosphate (K₂HPO₄) is a water-soluble inorganic salt commonly used in biochemical and molecular biology applications as a buffering component. It dissolves readily in water and provides alkaline phosphate buffering capacity, often used with other phosphate salts to prepare buffers of precise pH.
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Dipotassium phosphate (anhydrous) | K₂HPO₄ | 174.18 g/mol | 7758-11-4 |
| Dipotassium phosphate trihydrate | K₂HPO₄·3H₂O | 228.22 g/mol | 16788-57-1 |
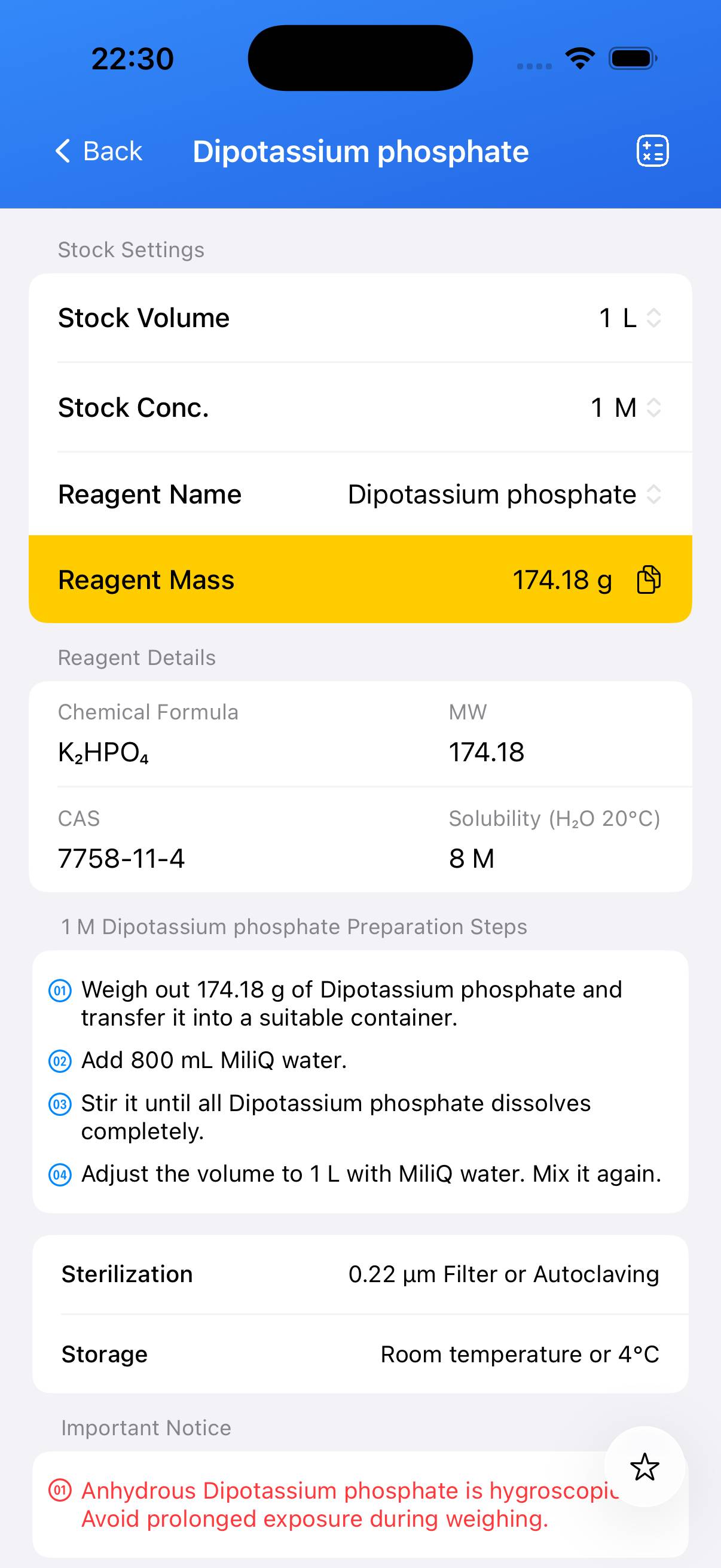
Preparation of 1 M Stock Solution
To prepare 1 L of 1 M dipotassium phosphate stock solution:
- Weigh the appropriate mass of dipotassium phosphate. For anhydrous K₂HPO₄, dissolve 174.18 g; for the trihydrate form (K₂HPO₄·3H₂O), adjust the mass to reflect the correct molarity.
- Transfer the solid into a suitable beaker or flask and add ~800 mL of deionized or distilled water. Stir until fully dissolved.
- Once dissolved, transfer to a 1 L graduated cylinder and add deionized water up to the 1000 mL mark. Mix thoroughly.
- The resulting solution should be clear and colorless. This 1 M stock can be used directly or mixed with other phosphate stocks to prepare buffers of specific pH.
Notes and Precautions
- Dipotassium phosphate is hygroscopic; minimize exposure to air when weighing.
- Add the solid to only part of the final water volume first, then dilute to the final volume to ensure accurate concentration.
- Use appropriate personal protective equipment (gloves, lab coat, goggles).
Storage and Applications
- Store the prepared 1 M stock at room temperature or 4 °C when not in use.
- This stock solution is a key component for preparing phosphate buffer systems at desired pH values when combined with other phosphate salts such as monopotassium phosphate.
Preview