Calcium chloride Stock Solution
This guide describes the preparation of Calcium chloride Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Calcium Chloride
Calcium chloride is a salt of calcium and chlorine widely used in biological and chemical laboratories as a source of calcium ions and for preparing ionic solutions. Calcium chloride is available in anhydrous and various hydrated forms, all of which can be used to prepare stock solutions, but hydrates such as the dihydrate are easier to handle due to hygroscopicity of the anhydrous form.
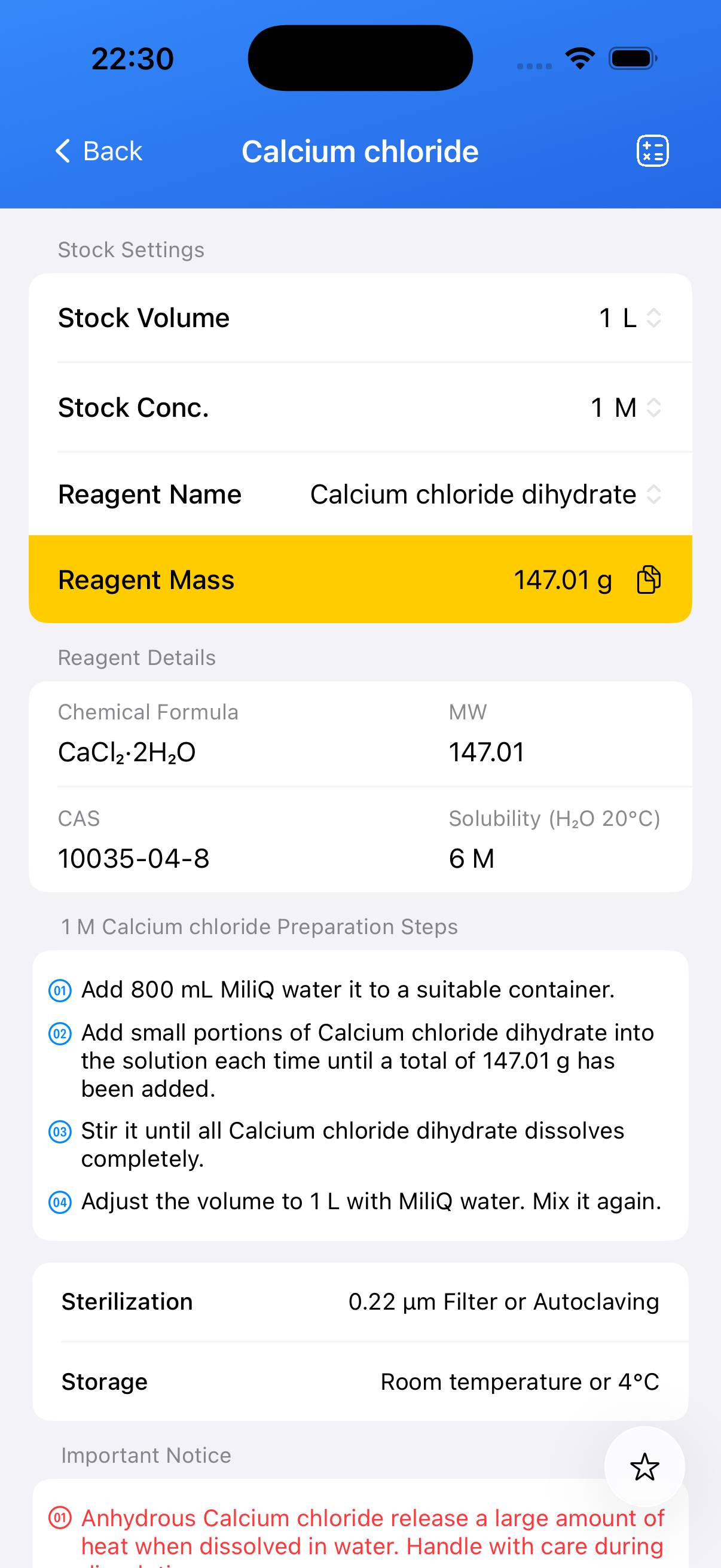
Chemical Information
| Name | Molecular Formula | Molecular Weight | CAS |
|---|---|---|---|
| Calcium chloride dihydrate | CaCl₂·2H₂O | 147.01 g/mol | 10035-04-8 |
| Calcium chloride tetrahydrate | CaCl₂·4H₂O | 183.05 g/mol | 25094-02-4 |
| Calcium chloride hexahydrate | CaCl₂·6H₂O | 219.08 g/mol | 7774-34-7 |
| Calcium chloride (anhydrous) | CaCl₂ | 110.98 g/mol | 10043-52-4 |
Preparation of 1 M Calcium Chloride Solution
To prepare 1 L of 1 M calcium chloride solution using calcium chloride dihydrate:
- Weigh 147.02 g of calcium chloride dihydrate (CaCl₂·2H₂O).
- Place the solid in a beaker or flask and add ~800 mL of deionized water. Stir until fully dissolved.
- After complete dissolution, transfer to a 1 L graduated cylinder and add deionized water to bring the final volume to 1000 mL.
- The resulting solution should be clear and colorless. This 1 M calcium chloride stock can be used for media preparation, ionic solutions, or other biological applications.
Notes and Precautions
- Calcium chloride dissolves exothermically in water; add the solid slowly and avoid splashing.
- Anhydrous CaCl₂ is highly hygroscopic and difficult to weigh accurately; hydrated forms are preferred.
- Use appropriate personal protective equipment (gloves, goggles, lab coat).
Storage and Usage
- Store the prepared 1 M calcium chloride solution at room temperature or refrigerated.
- This stock solution is widely used in buffer preparation, media formulation, and as a reagent in molecular biology and cell culture workflows.