Preparation Borate Stock Solution
This guide describes the preparation of Borate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Borate (Boric Acid, H₃BO₃) Solution
Borate typically refers to boric acid (H₃BO₃) solutions or borate buffers used in biochemical and analytical assays. Boric acid dissolves readily in water and can be used as a stock reagent for preparing buffers with defined pH. Typical borate buffers in the pH 8–9 range are prepared by neutralizing boric acid with a base such as sodium hydroxide.
Objective
Preparation of a boric acid stock solution and optionally a borate buffer.
Composition
- Boric acid (H₃BO₃) aqueous stock solution or borate buffer of desired pH
Required Materials
- Boric acid solid (H₃BO₃)
- Sodium hydroxide solution (for pH adjustment when preparing borate buffer)
- Deionized or distilled water
- Analytical balance
- Beakers, flasks, stirring rod or magnetic stirrer
- Volumetric flask or graduated cylinder
- pH meter or pH indicators
- Personal protective equipment (gloves, goggles, lab coat)
Procedure – Boric Acid Stock
- Step 1
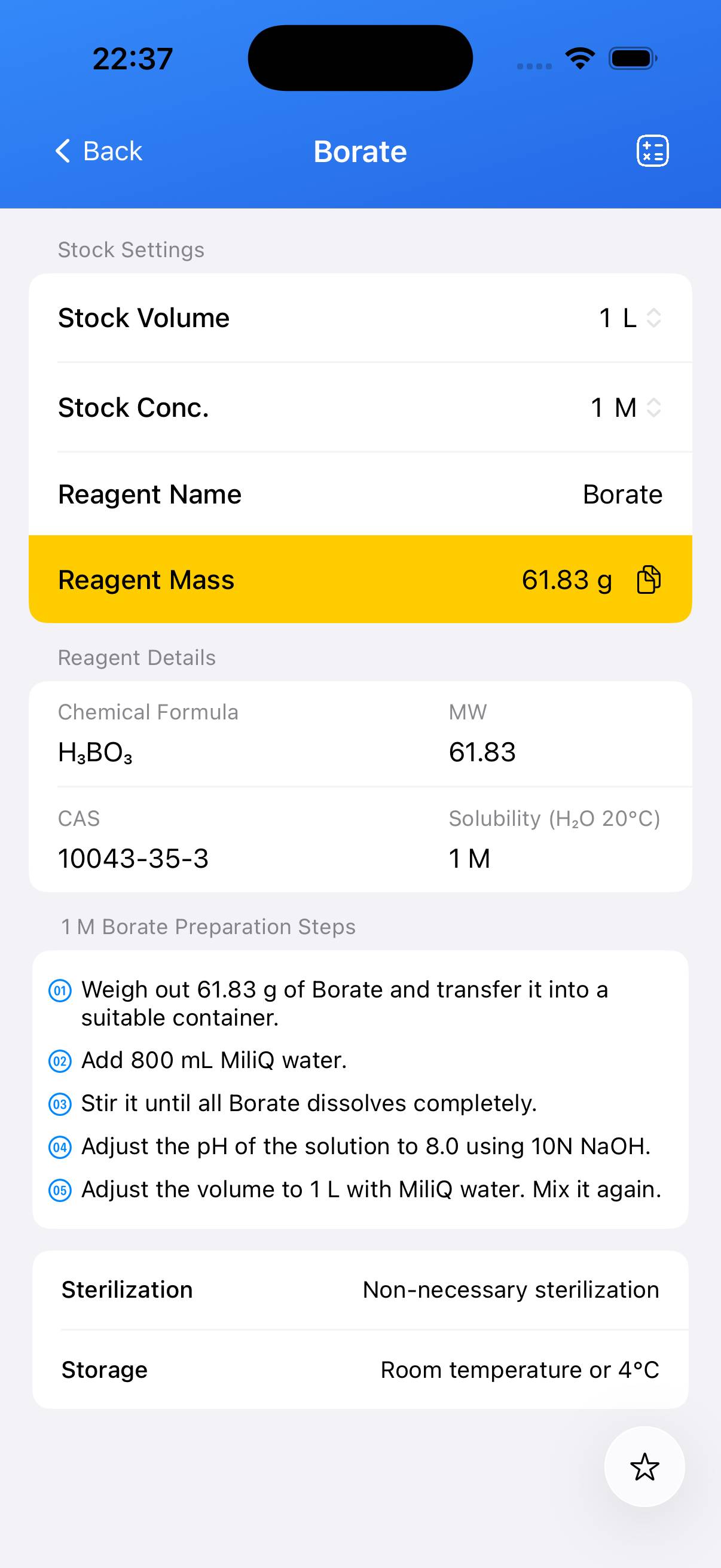
Choose the target concentration of boric acid stock. For example, to prepare a **0.5 M** stock: weigh **30.92 g** boric acid and transfer to a beaker with ~800 mL deionized water. - Step 2
Stir with gentle heat as necessary until the solid is completely dissolved. Allow to cool if heated. - Step 3
Transfer the solution to a 1 L volumetric flask and add deionized water to the mark. Mix thoroughly. - Step 4 (Optional)
For biological applications, filter sterilize through a **0.22 µm** membrane into a sterile container.
Procedure – Borate Buffer (e.g., pH 8–9)
- Step 1
Prepare the boric acid stock solution (e.g., 0.5 M). - Step 2
Slowly add a standardized NaOH solution while stirring and monitor the pH with a calibrated meter until your target pH (e.g., ~8.5–9.0) is reached. - Step 3
Transfer to a volumetric flask and adjust to final volume with deionized water.
Note: Borate buffers are typically used in the alkaline range near the pKa of boric acid (~9.24) and are applied in biochemical assays and electrophoresis buffer systems. Always label the stock and buffer solutions with concentration, pH, and preparation date, and store sealed at room temperature.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Borate (Boric acid) | H₃BO₃ | 61.83 | 10043‑35‑3 |