Ammonium chloride Stock Solution
This guide describes the preparation of Ammonium chloride Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Ammonium Chloride (NH₄Cl)
Ammonium chloride (NH₄Cl) is a white crystalline inorganic salt with high solubility in water, commonly used in biochemical and analytical laboratory settings. It dissociates into ammonium (NH₄⁺) and chloride (Cl⁻) ions in aqueous solution.
Objective
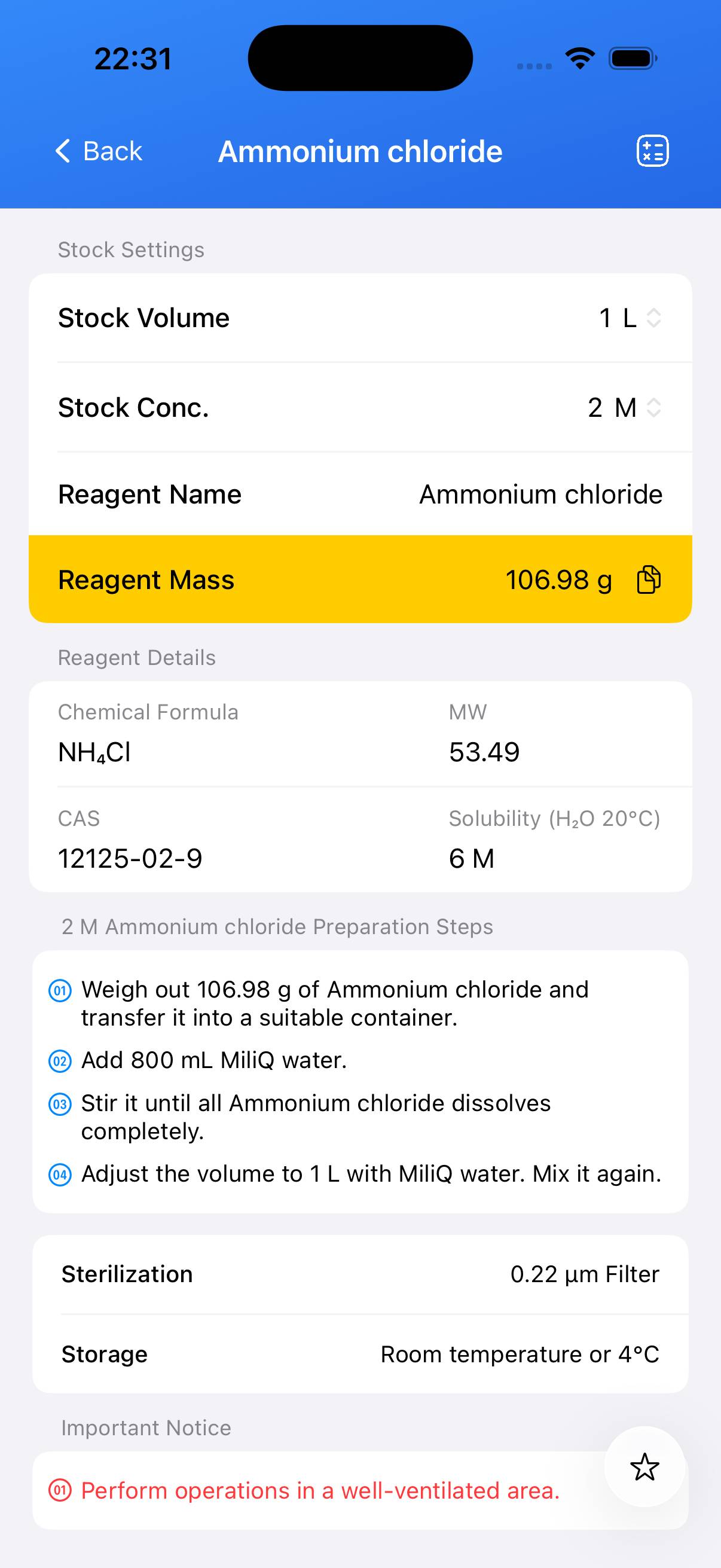
Preparation of 1 L of 1 M Ammonium Chloride stock solution.
Composition
- 1 M Ammonium chloride (NH₄Cl) in water
Required Materials
- Ammonium chloride (NH₄Cl)
- Deionized / distilled / Milli‑Q water
- Measuring cylinder or volumetric flask (1 L)
- Balance for solid weighing
- Magnetic stirrer or manual mixing device
Procedure
Use appropriate personal protective equipment (lab coat, gloves, goggles) and observe your institutional laboratory safety standards throughout the procedure.
- Step 1
Accurately weigh 53.49 g of NH₄Cl (Molecular Weight: 53.49 g/mol) for a 1 M solution. Place it into a suitable container. - Step 2
Add approximately 800 mL of deionized water and stir until fully dissolved. - Step 3
After complete dissolution, transfer to a 1 L volumetric flask (if used) and bring the final volume to 1 L with deionized water. Mix thoroughly.
Note: If a different final volume or molarity is needed, adjust the mass of NH₄Cl proportionally using the molarity equation.
(Molarity = moles/volume).
Sterilization and Storage
Aqueous NH₄Cl stock solutions can be filtered through a 0.22 µm membrane for sterilization if required for biological applications. Solutions are typically stable at 4 °C for several weeks; for long‑term storage, aliquot and freeze at −20 °C to avoid contamination.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Ammonium chloride | NH₄Cl | 53.49 | 12125‑02‑9 |