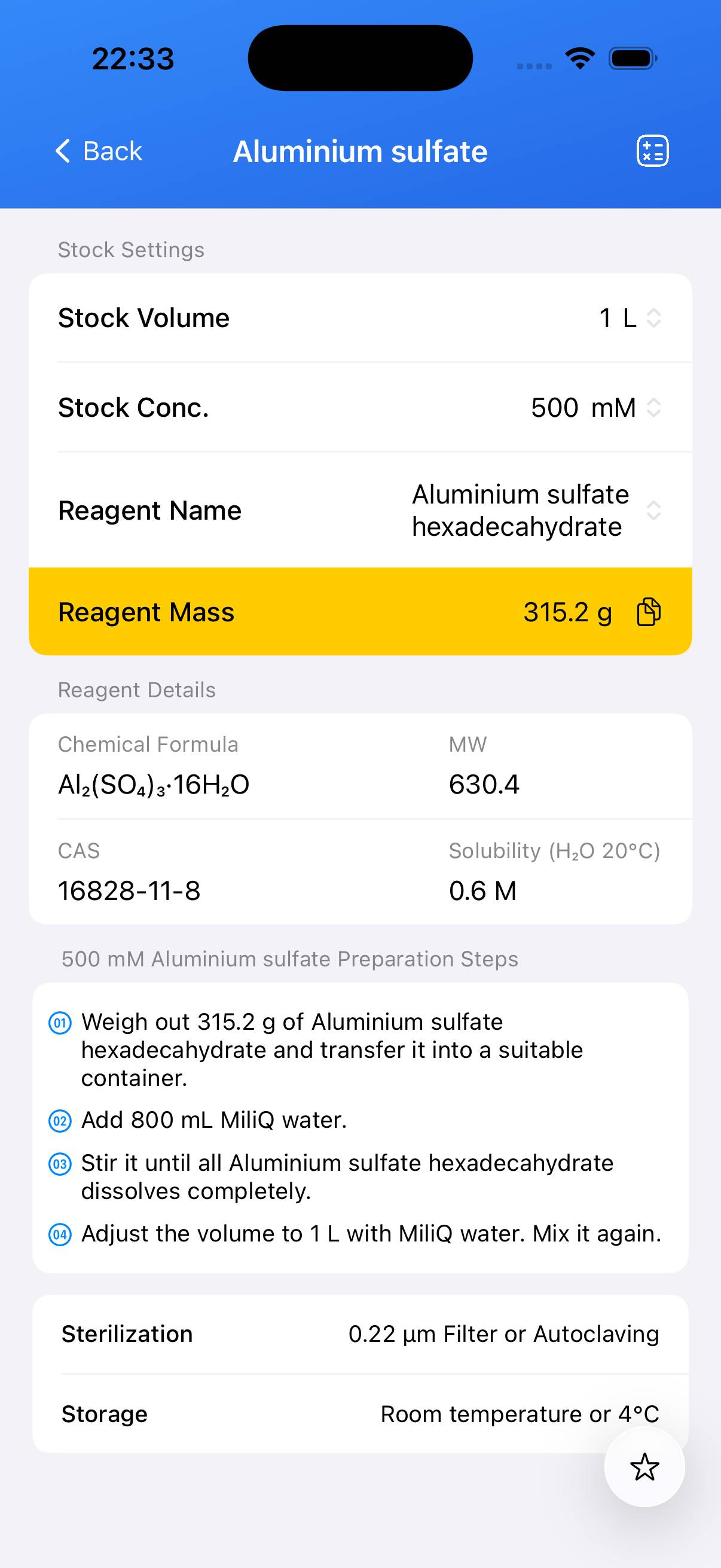
Aluminium sulfate Stock Solution
This guide describes the preparation of Aluminium sulfate Stock Solution at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Stock Solution Preparation Guide – Aluminium Sulfate (Al₂(SO₄)₃) Solution
Aluminium sulfate is commonly available in hydrated forms such as hexadecahydrate (Al₂(SO₄)₃·16H₂O) and octadecahydrate (Al₂(SO₄)₃·18H₂O) or as the anhydrous salt. In laboratory settings, the octadecahydrate form is often used to prepare defined‑concentration stock solutions because of its stable crystalline state.
Objective
Preparation of a defined concentration stock solution of aluminium sulfate (e.g., 1 M).
Composition
- Aluminium sulfate (Al₂(SO₄)₃) aqueous solution at the desired molarity
Required Materials
- Aluminium sulfate octadecahydrate (Al₂(SO₄)₃·18H₂O)
- Deionized or distilled water
- Analytical balance
- Beaker or flask
- Volumetric flask or graduated cylinder
- Magnetic stirrer or glass stirring rod
- Personal protective equipment (gloves, goggles, lab coat)
Procedure
- Step 1
Decide the target concentration (e.g., 1 M). For a 1 M solution of aluminium sulfate octadecahydrate: calculate and weigh the required mass (for 1 L, ~666.44 g Al₂(SO₄)₃·18H₂O). - Step 2
Add the weighed aluminium sulfate to ~700 mL of deionized water in a beaker. Place on a magnetic stirrer and stir until completely dissolved. Gentle warming (not exceeding 40 °C) may help dissolution. - Step 3
Transfer the dissolved solution to a volumetric flask. Rinse the beaker with small amounts of deionized water and add the rinses to the volumetric flask. Add water to bring the solution to the final volume (e.g., 1 L). Mix thoroughly. - Step 4 (Optional)
For sterile applications, filter the solution through a 0.22 µm membrane filter into a sterile container or autoclave if appropriate for your use case.
Note: Hydrated salts are preferable for accurate weighing. Anhydrous aluminium sulfate is hygroscopic and unsuitable for precise solution preparation without accounting for water uptake. Store the finished stock in a sealed container with a clear label indicating concentration and date.
Available Reagents
| Name | Molecular Formula | MW | CAS |
|---|---|---|---|
| Aluminium sulfate hexadecahydrate | Al₂(SO₄)₃·16H₂O | 630.4 | 16828‑11‑8 |
| Aluminium sulfate octadecahydrate | Al₂(SO₄)₃·18H₂O | 666.43 | 7784‑31‑8 |
| Anhydrous aluminium sulfate | Al₂(SO₄)₃ | 342.15 | 10043‑01‑3 |