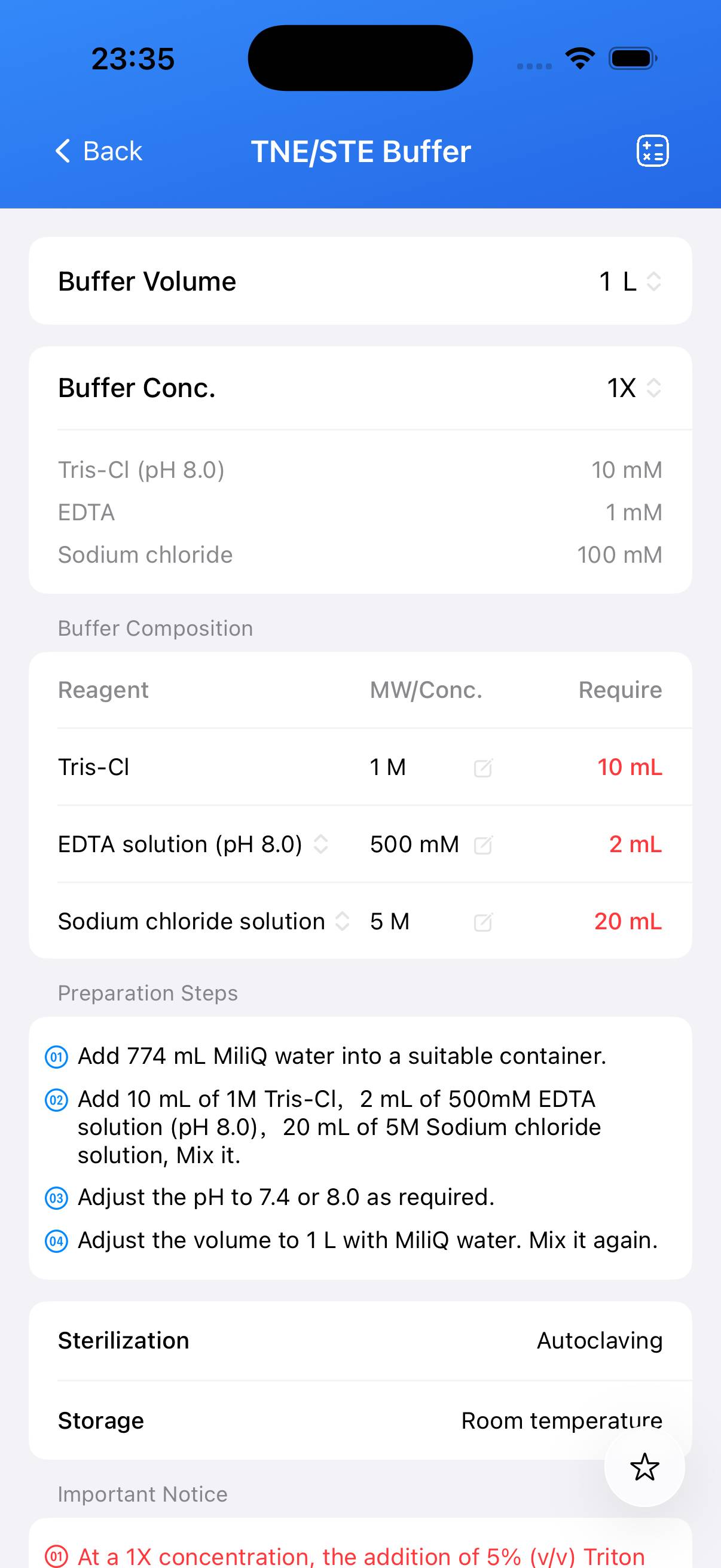
Preparation TNE/STE Buffer
This guide describes the preparation of TNE/STE Buffer at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
TNE buffer is commonly used in molecular biology for nucleic acid and protein applications.
TNE – Composition
| Name | Formula | Concentration | CAS |
|---|---|---|---|
| Tris-Cl (pH 8.0) | C₄H₁₁NO₃ | 10 mM | 77-86-1 |
| EDTA | C₁₀H₁₆N₂O₈ | 1 mM | 60-00-4 |
| Sodium chloride | NaCl | 100 mM | 7647-14-5 |
Sterilization & Storage
- Sterilization: Autoclave
- Storage: Room temperature
Tips
- At a 1X concentration, the addition of 5% (v/v) Triton X-100 yields STET buffer.
- EDTA (free acid) is extremely difficult to dissolve. It is recommended to prepare an EDTA stock solution first.
- The use of EDTA solution or EDTA·2Na·2H₂O is recommended.
Preview