Preparation Tris-acetate-EDTA Buffer
This guide describes the preparation of Tris-acetate-EDTA Buffer at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Tris-acetate-EDTA (TAE) buffer is a commonly used electrophoresis buffer for nucleic acid analysis, providing a stable pH environment and effective chelation of divalent metal ions.
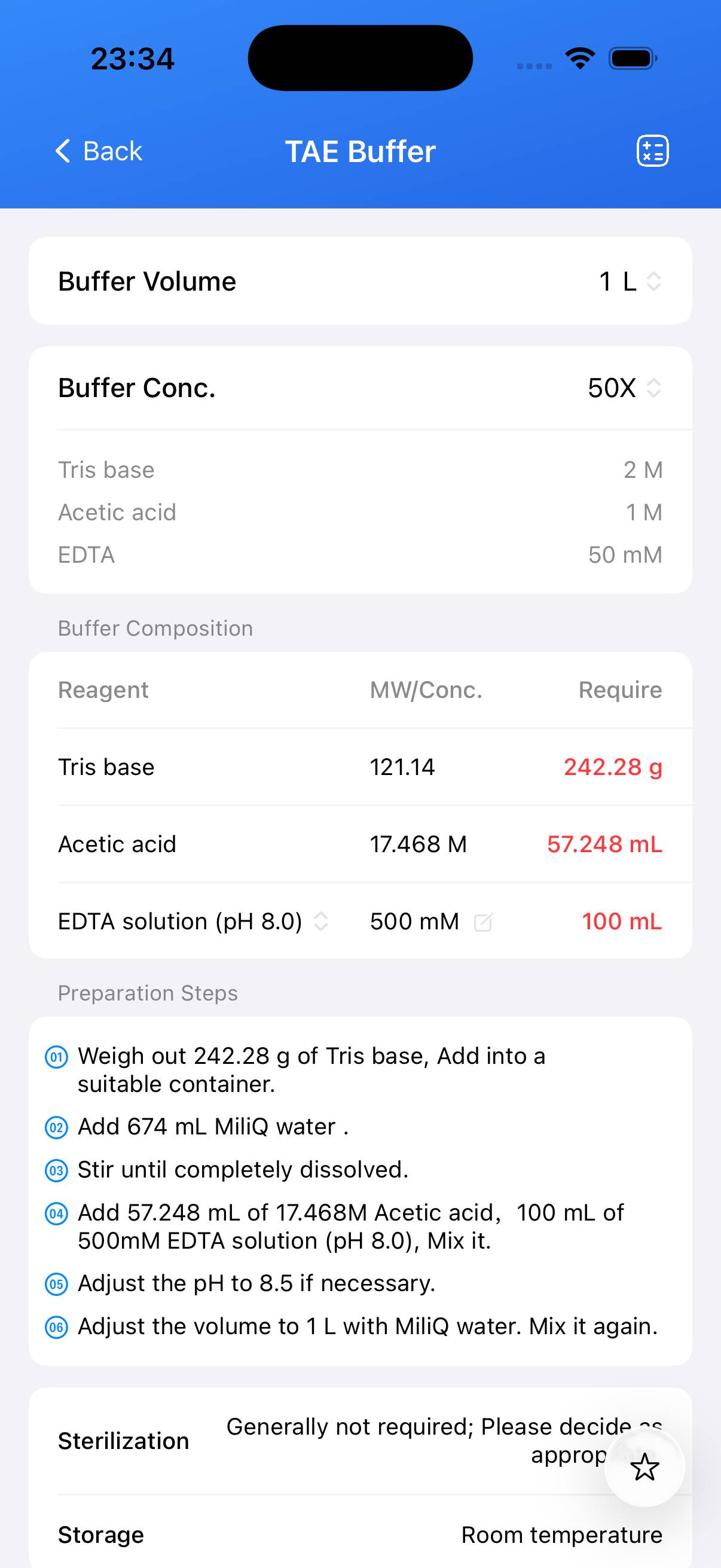
Tris-acetate-EDTA (TAE) Buffer – 1X Working Solution Composition
| Name | Formula | Concentration (1X) | CAS |
|---|---|---|---|
| Tris base | C₄H₁₁NO₃ | 40 mM | 77-86-1 |
| Acetic acid | CH₃CO₂H | 20 mM | 64-19-7 |
| EDTA | C₁₀H₁₆N₂O₈ | 1 mM | 60-00-4 |
Applications of Tris-acetate-EDTA (TAE) Buffer
TAE buffer is widely used for agarose gel electrophoresis of DNA and RNA. Tris provides buffering capacity, acetic acid adjusts ionic strength and pH, and EDTA chelates divalent cations such as Mg²⁺, inhibiting nuclease activity. TAE buffer offers good resolution for large DNA fragments and is commonly used in cloning, restriction analysis, and routine nucleic acid separation.
Preparation Tips, Sterilization, and Storage
- The use of EDTA solution or EDTA·2Na·2H₂O is recommended.
- Some studies have shown that buffers prepared with EDTA (free acid) generate less heat during electrophoresis, but require longer preparation time.
- EDTA (free acid) is extremely difficult to dissolve and requires pH 8.0. It is recommended to prepare an EDTA stock solution first.
- The molar concentration of glacial acetic acid (100% acetic acid) is approximately 17.5 M.
- If a small amount of precipitate forms, it can be redissolved by placing the solution in a 37°C water bath and does not affect use. If precipitate remains after water bath treatment, discard the solution.
- Sterilization: usually not required; select as needed.
- Storage: room temperature.