Preparation Hank's Balanced Salt Solution (HBSS)
This guide describes the preparation of Hank's Balanced Salt Solution (HBSS) at a defined molarity for laboratory use.
The following content was generated by AI and has not been strictly verified; it may contain inaccuracies. All information in the BioCalculator App has been manually curated and carefully validated. You can download the App to view it.
Hank’s Balanced Salt Solution (HBSS) is a balanced salt buffer widely used in cell biology to maintain physiological osmotic pressure and pH during washing, transport, and short‑term maintenance of cells and tissues. It provides water and essential inorganic ions required for cell viability.
Hank’s Balanced Salt Solution – Composition
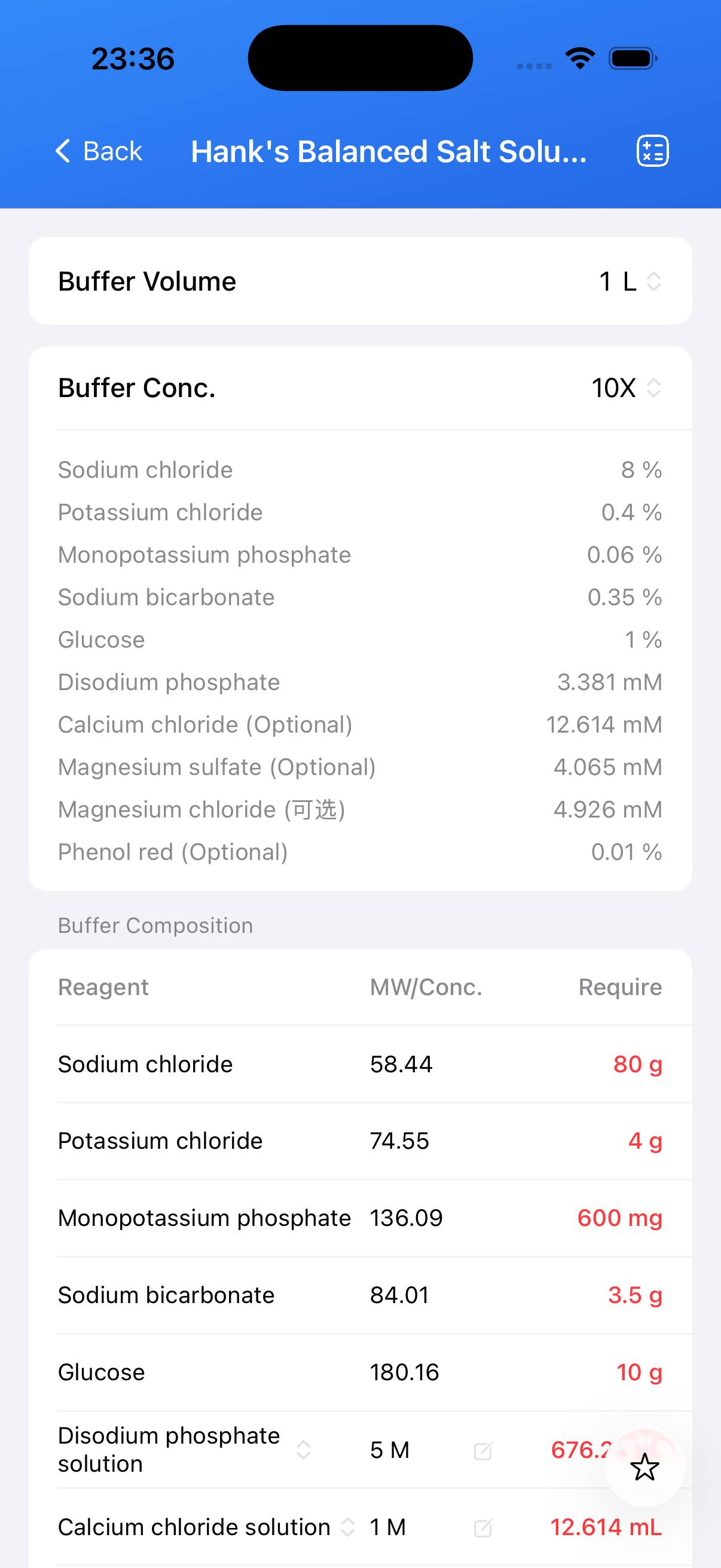
| Name | Formula | Concentration | CAS |
|---|---|---|---|
| Sodium chloride | NaCl | 0.8% (w/v) | 7647‑14‑5 |
| Potassium chloride | KCl | 0.04% (w/v) | 7447‑40‑7 |
| Monopotassium phosphate | KH₂PO₄ | 0.006% (w/v) | 7778‑77‑0 |
| Sodium bicarbonate | NaHCO₃ | 0.035% (w/v) | 144‑55‑8 |
| Glucose | C₆H₁₂O₆ | 0.1% (w/v) | 50‑99‑7 |
| Disodium phosphate | Na₂HPO₄ | 338.12 µM | 7778‑77‑0 |
| Calcium chloride (Optional) | CaCl₂ | 1.26144 mM | 10035‑04‑8 |
| Magnesium sulfate (Optional) | MgSO₄ | 406.5 µM | 10034‑99‑8 |
| Magnesium chloride (Optional) | MgCl₂ | 492.6 µM | 7786‑30‑3 |
| Phenol red (Optional) | C₁₉H₁₄O₅S | 0.001% (w/v) | 143‑74‑8 |
Sterilization & Storage
- Sterilization: 0.22 μm filtration to remove microbes (do not autoclave because of glucose content).
- Storage: Room temperature (protect from light).
Tips & Notes
- The addition of 20 mM HEPES yields HHBS Buffer.
- This solution contains glucose and must not be autoclaved.
- Precipitation may occur due to calcium ions. Warm in a water bath until the precipitate dissolves; if excessive precipitation forms, discard the solution.
- Because the NaHCO₃ content is low, this solution is not suitable for use in a 5% CO₂ incubator environment; it will rapidly become acidic under CO₂.
- Mg²⁺ may be supplied using magnesium sulfate alone (0.098 g/L) or in combination with magnesium chloride; choose as appropriate.
- Anhydrous magnesium sulfate and calcium chloride are highly hygroscopic and hard to weigh; their use is not recommended.
- Anhydrous calcium chloride releases heat upon contact with water; handle with care.
Preview